Medication error due to switching of vials at HUS on April 2023
Description of the incident
A patient came in for a planned day surgery procedure. S/he was to be given a sedative before the procedure. A physician gave a nurse a verbal prescription for this. The nurse anaesthetist dispensed medicine from a vial into a syringe and administered it to the patient. The patient's condition rapidly deteriorated after the administration of the medicine. S/he developed cardiovascular symptoms, and the planned procedure was postponed. It was later discovered that the patient had been mistakenly administered a high dose of a medicine used to treat heart and circulatory system dysfunction, which affects vital functions, instead of a sedative. The patient received appropriate treatment and recovered from the medication error.
The physician checked the vials of medicine administered to the patient from the medical waste bin after the incident. A vial of medicine affecting the heart and circulatory system, which was not the medicine the physician had prescribed during the preparation for the procedure, was found from the medical waste (see Fig. 1). This confirmed the conclusion that the serious consequences to the patient were caused by the wrong medicine. The medication error was therefore verified.

Underlying factors
Two surgical nurses, a nurse anaesthetist and a physician were present. The working environment in the operating theatre was normal. The planned procedure was also normal. There was no exceptional urgency at the time of the incident.
Medicines needed in the operating theatre were kept in medical basket carts. In the carts, medicines had been divided into compartments by pharmacotherapeutic group (see Fig. 2). In this case, the medicines that got mixed up were in adjacent compartments. The vials were similar in appearance in terms of colour, shape and size. The coloured stickers on the vials resembled each other. The size of the vial of the prescribed medicine was 4 ml and that of the wrong medicine 5 ml.
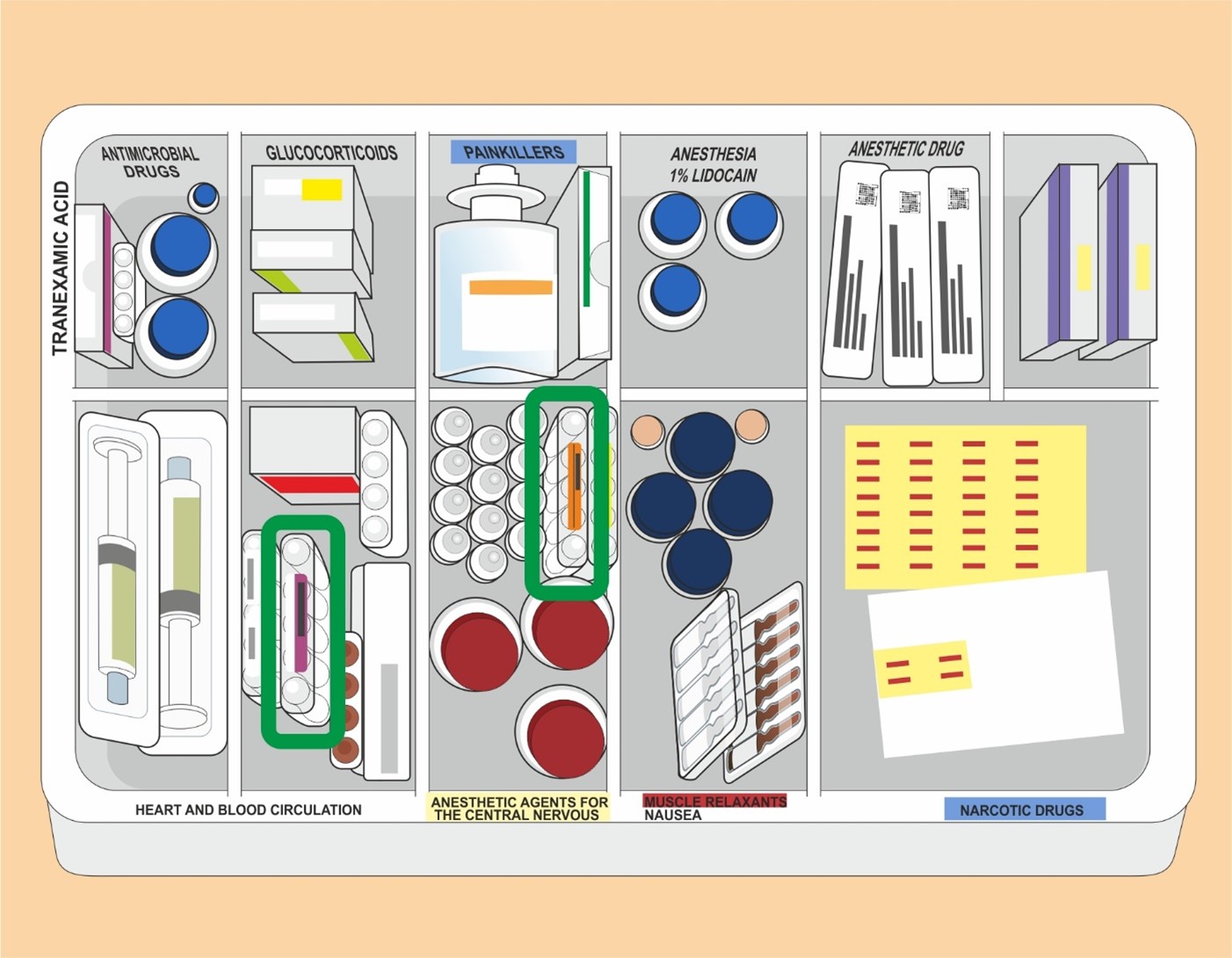
According to HUS guidelines, a double check must be carried out during the preparation and administration of medicine before administration. The administration of medicine is prescribed by a physician, and the medication is administered either by a physician or a nurse. No double check was performed when administering the medicine. The wrong medicine was dispensed from the vial into a syringe and administered to the patient intravenously.
According to the HUS guideline, if a double check cannot be carried out by two persons (e.g. due to staffing constraints), it is to be carried out by one person. A double check by one person involves checking the correctness of the prescription, first when the medicine is prepared for use and the second time before the medicine is administered to the patient.
In HUS, barcodes for medicines used in the operating theatre are not entered into the Apotti system. The operating theatre facilities do not always allow for the equipment required by the barcode system (printer and computer), as single vials do not have a machine readable barcode. Thus, for the barcode system to be fully realibale, a separate printing and taping of a barcode would be required. Furthermore, the use of the bar code system would complicate the preparation of the medecine in emergency situations. However, in emergency situations requiring immediate action, the barcode can be bypassed.
The colour coding of medicines is based on choices made by the manufacturer and marketing authorisation holder. In terms of colour coding, HUS currently uses colour-coded syringe labels provided by Yliopiston Apteekki pharmacy (YA), the colour coding of which complies with international recommendations (e.g. the ISO standards). There is currently no requirement from the medication safety authorities to harmonise the colour coding of vials.
The manufacturer of medicines can change frequently, resulting in changes in the size, concentration and labelling of the medicines used in the operating theatre. This contributes to the risk of a medication error.
At the time of the incident, the medication management plans of the responsible units were in the process of being updated.
According to reported adverse events by HUS, medication safety is compromised for around one in every ten patients (11% of all patients in HUS). Based on the reported adverse events, medication errors in the operating room seem to be less frequent than in other activities. All in all, the severe adverse events reported for operating theatres between 1 January 2022 and 19 April 2023 amounted to 0.02% of approximately 87,000 operations.
Observations
As a rule, medicines used in the operating theatre are always life-threatening if administered incorrectly. Double-checking during the administration of medication is absolutely necessary, and there are separate guidelines for this. The working environment must support concentration on the administration of medication. The placement of the vials next to each other in the medical basket cart and the similar size and colouring of the vials increased the risk of the medicines getting confused, and these were factors that increased the risk of a safety deviation. The instructed double check was not carried out in connection with the administration of the medicine.
The risk of medication errors is reduced by planned and agreed placement of pharmaceutical preparations and the use of harmonised coloured labels. These contribute to reducing the possibility of mixing the medicines. The high turnover of pharmaceutical preparations increases the risk of a medication error.
Following medication guidelines, using a checklist and double-checking when preparing and administering medicines assist in preventing medication errors.
Following the incident, HUS has conducted an internal investigation. On the basis of the investigation, HUS further specified its medication guidelines, taking into account the placement of medicines and the training of staff. HUS is also considering the use of new technical solutions (a smart anaesthesia table) to improve patient safety. A guideline to harmonise the labelling of medicines was being prepared at the time of the incident.
In the future, it should be assessed whether guidance from the pharmaceutical safety authority could influence the harmonised colour coding of medicines. When preparing criteria for calls for tenders for pharmaceutical products, particular attention must be paid to pharmaceutical safety.
An adverse reaction notification should be filed for all errors in medication, and all incidents should be investigated to learn from the mistakes. The lessons learned from the investigation of medication errors should be disseminated throughout the industry.
This report has been prepared under the powers conferred for a preliminary investigation. Safety Investigation Authority Finland will not initiate an actual safety investigation into the incident.
Further information: Chief Safety Investigator Hanna Tiirinki (Safety Investigation Authority Finland), tel. +358 2951 50747
Published 15.8.2023
